How electroconvulsive therapy affects inflammation and new brain cells
- Juliette Giacobbe

- Aug 6, 2020
- 6 min read
Updated: Mar 23, 2023
More than 80 years since it was first used as a psychiatric treatment, electroconvulsive therapy (ECT) still conjures up very strong images.
The main topic I want to discuss in this blog is how ECT acts inside the brain but first, let’s talk about its clinical use and the stigma surrounding it.
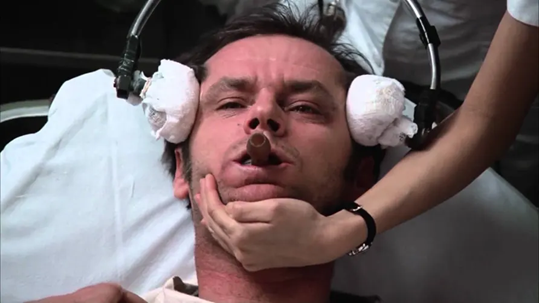
Because when we mention ECT, we think of Jack Nicholson in One Flew Over the Cuckoo’s Nest, strapped against his will to a table by a tyrannical nurse, conscious and awake through the entire procedure.
We think of Sylvia Plath’s The Bell Jar, where Esther has a frightening experience of unmodified ECT, that is without anaesthesia, before later feeling “at peace” after modern modified ECT.
Often, we think of images we encounter in the media, which tend to depict ECT at its beginnings.
The media is right about one thing: ECT does stimulate the brain via electrodes placed on the head to induce a seizure. But it rarely represents the reality of ECT today.
Today, ECT is used to treat depression. It helps relieve symptoms in cases of severe depression or in situations considered to be life-threatening for the patient.
It is not used without the patient’s consent. The procedure, its benefits and risks are explained by a doctor before making any decision.
General anaesthesia and muscle relaxants are used.
The health of patients is closely monitored during and after ECT.
There really are no reasons to keep the historically negative stigma surrounding ECT alive.
Although a more recent systematic review has raised doubts on some of the evidence we have so far, current guidelines on depression treatment considers modern ECT a well-established and safe treatment.
While it is never used as a first-line treatment, ECT has been demonstrated to be more effective than first-line pharmacological treatment, which is usually antidepressant medication. ECT is also a fast-acting treatment compared with antidepressants, which can take several weeks before improving symptoms.
For this reason, it is indicated for patients with severe depression and who are, in some cases, treatment-resistant, meaning that they do not respond to first-line treatment. Patients who use ECT in combination with common antidepressant drugs such as selective serotonin reuptake inhibitors (SSRIs), are also less likely to relapse and have another episode of depression.
For some treatment-resistant patients, ECT can be the difference between a recovery process and the risk of prolonged periods of severe depression. Such periods can lead to impaired quality of life and even life-threatening consequences, including suicidal acts and physical deterioration from lack of food and water intake.
In addition, the most common side-effects, disorientation and memory loss, are temporary most of the time. They also seem to be more common as the severity of depression increases, because this can lead to more significant memory and cognitive dysfunctions.
Even though there is evidence to demonstrate the treatment’s effectiveness and safety, curiously enough, scientists still have to solve the mystery of how ECT acts in the brain to lead to such quick improvements.
As ECT triggers a generalised seizure, many biological mechanisms could be involved and affected. However, some theories are more popular and more widely studied than others. One of these is a topic very dear to me, the neuroplasticity and neurogenesis theory. You will have read about this topic if you saw some of our previous blogs on omega-3 fatty acids and blood inflammation.
So get ready, we’re talking neuroscience.
Here are the basics: neuroplasticity refers to the ability of the brain to remodel connections and networks, in response to changes in the environment or to a disease or injury. Neurogenesis is the process whereby neural stem cells (aka brain-cells-to-be), which are present only in specific parts of the brain in adulthood, proliferate, differentiate, and integrate into the existing networks.
Neurogenesis is thus tightly associated with neuroplasticity because it determines how new neurons are born, mature and form connections within the circuitry.
Here too, the environment and biological processes can have a great influence because stem cells can proliferate at different rates and can differentiate into several types of brain cells, not all of which are neurons. For example, stem cells can also become astrocytes, brain cells which support neurons and can propagate inflammatory signals, amongst many other functions.
In adults, these processes mainly take place in a part of the brain called the hippocampus. This area is well-known for its established role in memory and learning. It is one of the focus areas in depression research because it plays a particular role in the cognitive aspects of the disorder, including the emotional memory dysfunctions.
And indeed, when patients with depression have an MRI scan, researchers see that the volume of their hippocampus is reduced. This indicates that hippocampal degeneration is involved in depression. Animal studies have long supported this and show that dysfunctions in hippocampal neuroplasticity and neurogenesis are associated with depression-like behaviour.
Where does ECT fit into all of this?
ECT has been able to reverse reduced hippocampal volume and is thought to increase the ability of neurons to respond to electric signals in the hippocampus, where immature cells (aka baby neurons) might be particularly sensitive.
So ECT comes in, boosts neurogenesis and plasticity then depression gets better? Of course not, that would be too easy.
Depression can be associated with elevated levels of inflammation, which you can read more about in these previous blogs. Treatment-resistant patients, in particular, are known for having higher levels of inflammation.
Right, but the problem with inflammation is that it typically is considered bad news for neurogenesis. Higher levels of inflammatory molecules cause reductions in proliferation of neural stem cells and lead to the manifestation of depressive-like symptoms, as shown in animal studies. This behaviour is also observed when the immune cells of the brain, called microglia, are activated.
Interestingly, ECT has been reported to increase markers of inflammation in the blood of patients and seems to decrease these only in the long-term.
Hmm, so how could ECT boost neurogenesis but also cause inflammation, which is known to impair neurogenesis?
That was our question too, so we decided to tackle this in our recent paper, published in the scientific journal Journal of Psychopharmacology, last month.
To get an answer, we left no stone unturned and searched for any study that measured the presence of any molecule related to neurogenesis and at the same time, the presence of any molecule related to inflammation or immune activation in the brain. This way, we could observe how both neurogenesis and inflammation were affected by ECT.
To be more precise, our review focused on studies using ECS (electroconvulsive shock), which is the method used to model ECT in animals: like human patients, they are completely anaesthetised and electrodes are placed on their head to induce a seizure.
The studies we found revealed that in the brain of these animals, ECS led to an increase in immune activation and inflammatory molecules, associated with an upsurge in growth and trophic factors, which are molecules promoting neurogenesis, in the first few hours.
Even more interestingly, many of the studies we found also reported that astrocytes were also more activated. In depression, astrocytes are usually dysregulated and atrophied, meaning that they decline and reduce in size, so this may be one mechanism explaining the therapeutic effects of ECT in patients.
However, studies showed that over time, the brain’s inflammatory response was dampened, while astrocytes remained activated and the proliferation of new brain cells continued, especially in the hippocampus (see our graph below).
Interestingly, this mirrors the clinical finding showing that ECT leads to an increase in inflammation directly after treatment but tends to slowly lower baseline inflammation, meaning right before ECT, over the course of the sessions.
It would then seem that an increase in immune activation initially helps with regulating neurogenesis processes. One of the hypotheses we formulated is that, considering that some molecules of the immune system can both heighten or dampen inflammation, they could actually benefit neurogenesis under the right circumstances.
Have these studies helped to solve the ECT enigma? Not completely, but they give us one more piece of the puzzle and tell us what research should investigate. For example, the fact that inflammation is one mechanism modulated by ECT might explain why this treatment option is effective in treatment-resistant patients, who often have increased levels of inflammation.
While writing this paper, we discovered that activation of the immune system and inflammation, which we originally thought would have negative consequences, did not cause damage to young brain cells after ECS overall.
Perhaps this can make us think about the prejudice against ECT. It’s easy to think of it as a simply crude and brutal procedure, unless we look at the facts.
Of course, it looks scary - it directly affects the brain, the organ we most strongly associate with who we are.
Of course, we would prefer to know exactly how ECT works on the brain.
But we know that ECT is effective. We know it is considered safe. If you had severe depression and antidepressants did not have an effect, would you not prefer to have at least an option?